FDA Experts Recommend Moderna Booster Dose of Covid-19 Vaccine
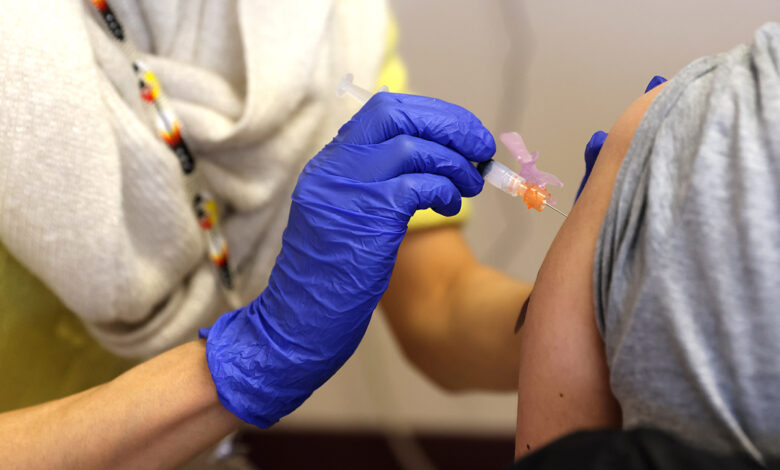
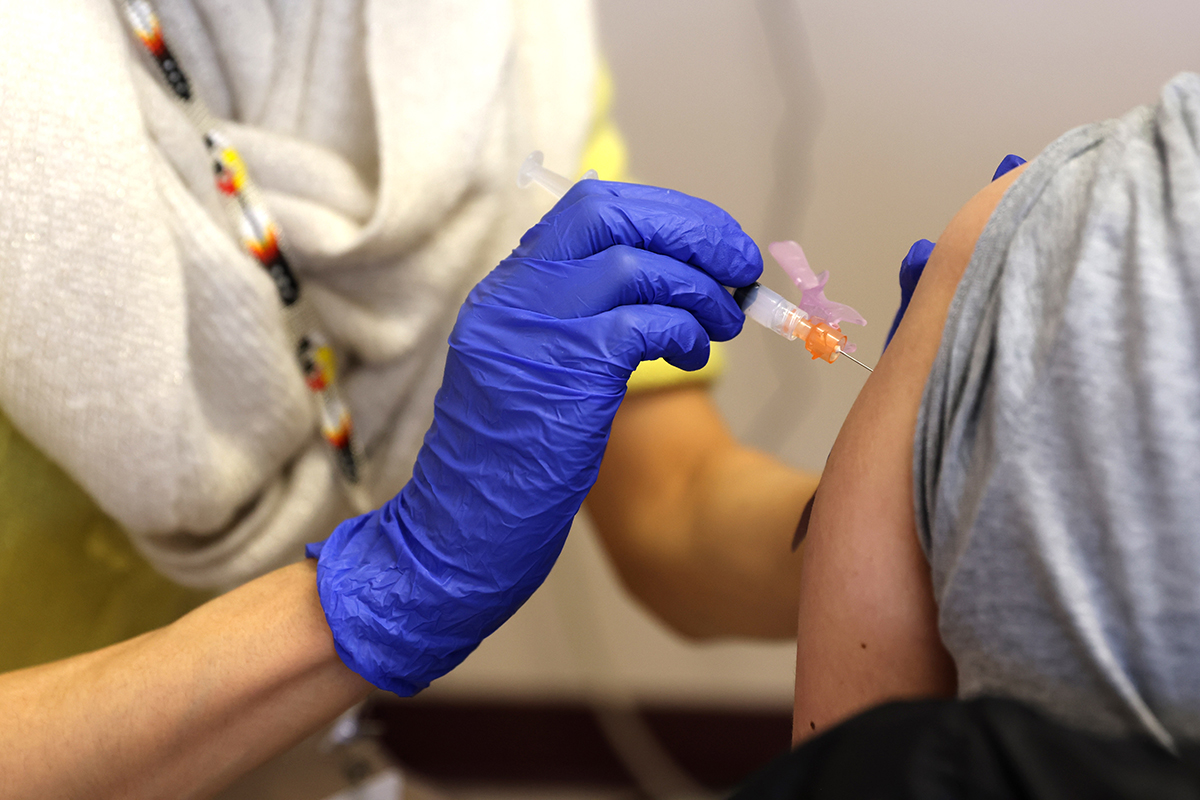
A key advisory committee to the Food and Drug Administration (FDA) unanimously recommended on Thursday to administer booster injections of the Covid-19 vaccine from Modern to people 65 and older and other vulnerable Americans, a crucial step before the United States can begin administering third doses of the Covid-19 vaccine from Modern to some of the more than 69 million people who originally received it.
The non-binding decision of the FDA’s Advisory Committee on Vaccines and Related Biologics would set the guidelines for Modern in line with third doses of the vaccine from Pfizer and BioNTech.
Those booster vaccines from Pfizer and BioNTech were licensed less than a month ago for a wide range of Americans, including the elderly, adults with underlying medical conditions, and those who work or live in high-risk settings such as healthcare workers and grocery stores.
While the agency has not always followed the advice of its committee, it often does.
A final FDA decision on the third dose of the Covid-19 vaccine from Modern could arrive in a few days.
The next step will occur when a CDC vaccine advisory committee votes on the FDA proposal.
If approval is recommended and supported by the CDC, booster vaccines Modern they could start immediately for eligible Americans who completed their vaccinations at least six months ago.
It may interest you:
CDC panel recommends third dose of Pfizer-BioNTech vaccine for many, including older people
.



