FDA Authorizes Moderna and Johnson & Johnson Covid-19 Booster Vaccines
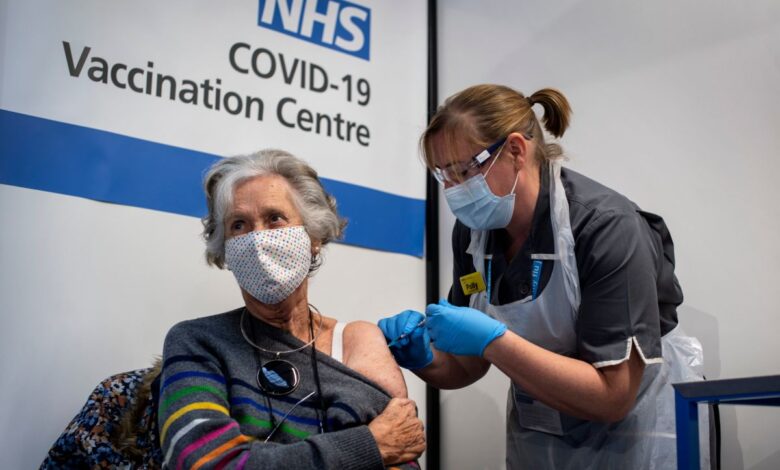
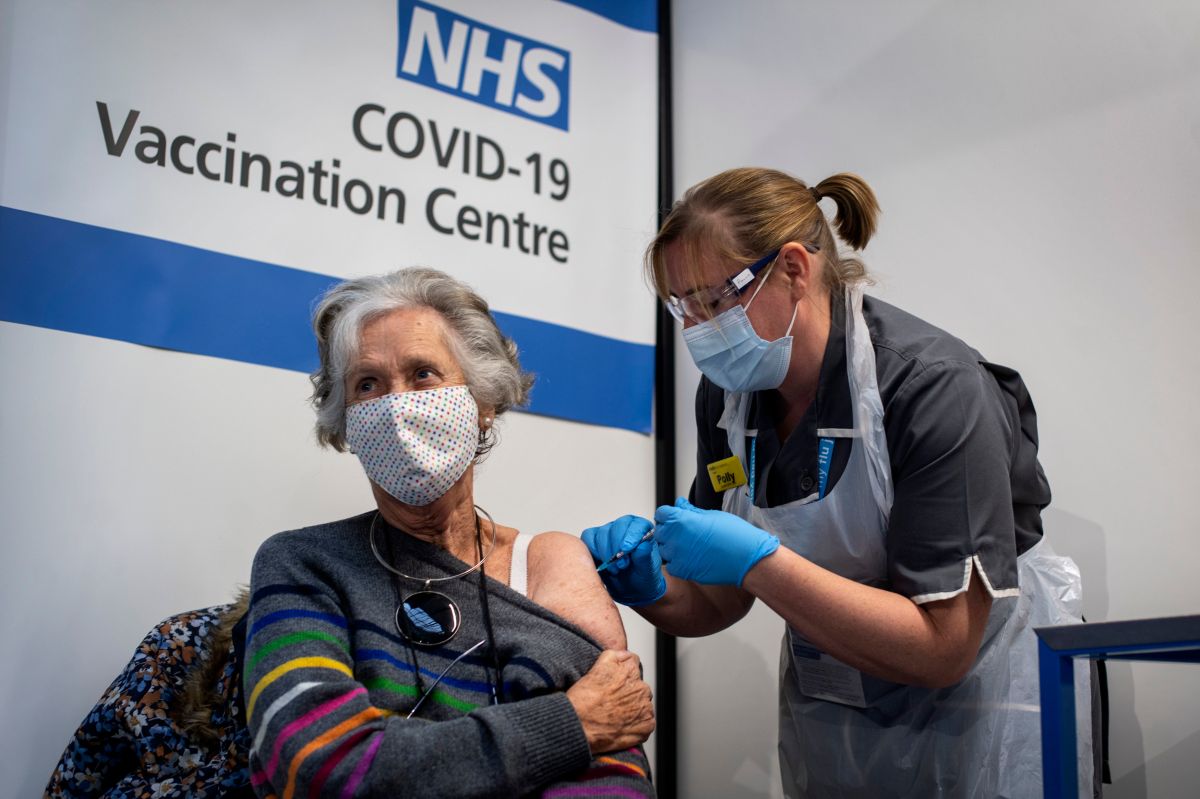
They want to increase the anti-covid vaccination rate.
Photo: Victoria Jones – Pool / Getty Images
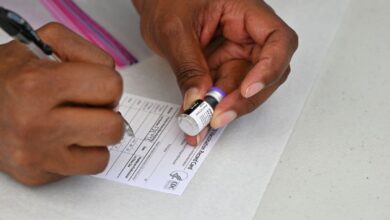
The Food and Drug Administration (FDA) authorized booster dose of both Covid-19 vaccines from Moderna and Johnson & Johnson on Wednesday and also said that any of the three licensed Covid-19 vaccines could be used as a booster in a “mix and match” approach.
The FDA granted an emergency use authorization for Moderna’s vaccine boosters for people fully vaccinated at least six months ago who are also at least 65 years old, or who are at least 18 years old and are at high risk of Covid-19 severe or have frequent institutional or occupational problems. and risk of exposure to coronavirus.
“The use of a single booster dose of the Janssen (Johnson and Johnson) COVID-19 vaccine can be given at least 2 months after completing the primary single-dose regimen to individuals 18 years of age or older,” he added. the FDA in a statement.
The vaccine advisers who make up the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) will meet Thursday to decide whether to recommend FDA clearance for Americans, and then the CDC director will decide whether to approve the ACIP recommendation.
Millions more people in the United States will soon be able to receive an additional injection of any coronavirus vaccine, regardless of their initial vaccination, a flexibility that comes along with the authorization Wednesday of Moderna and Johnson & Johnson booster vaccines. from federal regulators.
.